A cold medication made in India and sold in Iraq is tainted with toxic chemicals, a test commissioned by Bloomberg News shows, the latest in a series of alarming revelations about syrup medicines used by children around the world.
A bottle of Cold Out purchased at a pharmacy in Baghdad in March contains 2.1% ethylene glycol, according to Valisure LLC, an independent US laboratory. That’s about 21 times the widely accepted limit. The compound is lethal to humans in small amounts and played a role in mass child deaths caused by Indian-made cough syrups in Gambia and Uzbekistan last year.
Bloomberg shared the test results with the World Health Organization as well as Iraqi and Indian officials on July 8. The WHO told Bloomberg that it found Valisure’s test results to be “acceptable” and that it will issue an alert if the Iraqi government confirms the product was sold there. No public alert or recall has been announced yet.
Saif al-Bader, a spokesman for Iraq’s health ministry, said in an interview that the ministry has “strict regulations for the import, sale and distribution of medicines.” He declined to answer specific questions about Cold Out.
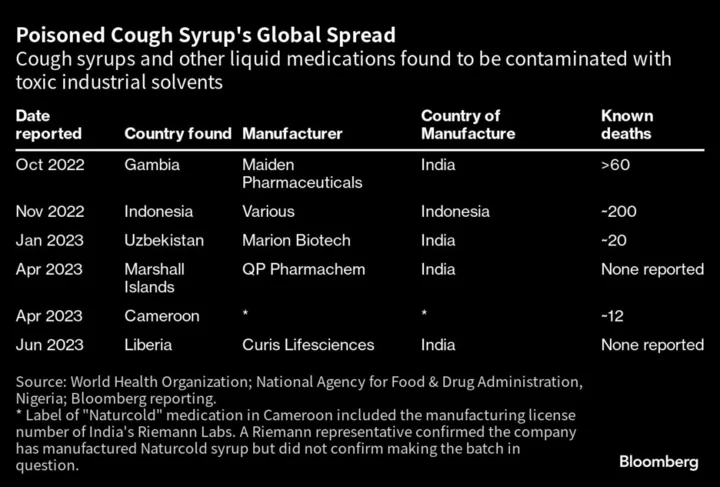
It’s the fifth time in a year that testing has found an Indian exporter’s drugs to contain excessive levels of ethylene glycol. In addition to the Gambia and Uzbekistan outbreaks, testing by government laboratories has identified other contaminated products in the Marshall Islands and Liberia, although there were no reported illnesses associated with those drugs.
The Cold Out label indicates it was made by Fourrts (India) Pvt. Ltd., a Chennai-based manufacturer that exports medicines to more than 50 countries, including the UK, Germany and Canada. A vice president there, Bala Surendran, said that Fourrts subcontracted the manufacture of Cold Out to another Indian company, Puducherry-based Sharun Pharmaceuticals Pvt. Ltd.
After Bloomberg’s inquiries, Fourrts tested a sample of Cold Out it had on hand and found it untainted, Surendran said. He said Indian regulators seized other samples from Sharun’s plant and that Fourrts hasn’t been informed of the results of those tests. Officials at the national drug agency and two local regulators either did not respond to requests for comment or said they had no information to share. Sharun executives did not respond to requests for comment.
The outbreak last year in Gambia killed more than 60 children, and the one in Uzbekistan killed about 20. The incidents raised fresh questions about the quality of drug exports from India, which is the largest generic drugmaker and calls itself the ”pharmacy of the world.”
The WHO said this month that a cough syrup blamed for 12 child deaths in Cameroon this year contained unsafe levels of diethylene glycol, a similar toxic compound. In that case, the medicine packaging doesn’t name a maker but bears the manufacturing license number of another Indian company.
Earlier this year, as part of an investigation into the global trade in unsafe drugs, Bloomberg purchased 33 samples of Indian-made syrups from pharmacies in Cambodia, Georgia, Ghana, India, Iraq and Kenya. The drugs were tested by New Haven, Connecticut-based Valisure using gas chromatography-mass spectrometry. The lab found four samples, all different brands, that contained either ethylene glycol, diethylene glycol, or both.
In considering whether a drug product contains unsafe levels of ethylene glycol or diethylene glycol, the WHO uses a guideline of 0.1%. Levels above that “would be considered non-compliant and therefore a health risk,” Rutendo Kuwana, head of the organization’s substandard medicines team, said in an email. Sarah Sheppard, a WHO spokeswoman, pointed to guidance from the US Food and Drug Administration that uses the 0.1% limit for tests of raw materials used in syrup production.
Valisure tested the Cold Out sample five times and found, on average, ethylene glycol content of 2.1% and diethylene glycol content of 0.25%. The diethylene glycol content is more than twice the limit. None of the other syrups with contaminants exceeded the 0.1% level.
Bloomberg provided WHO and Iraqi authorities with test results and the name and location of the Baghdad pharmacy where the syrup was purchased. The WHO’s Sheppard said in an email this week that Iraq continues “to attempt to source samples to confirm (or not) whether the product is in their country and where else it could be on sale. To raise a definitive alert, WHO and the Member State would need to be satisfied that it was on sale in a particular location.”
“We will issue an alert as soon as we have confirmation of the information from Iraq,” Sheppard continued.
Syrup medications consist of a small amount of active ingredient suspended in a watery solution. To cause the active ingredients to dissolve, manufacturers add a solvent such as propylene glycol — a harmless, clear, sweet-tasting liquid.
Ethylene glycol and diethylene glycol are chemically similar to propylene glycol but are cheaper and highly toxic, used in industrial applications such as antifreeze and brake fluid. Typically, contamination takes place when a chemical trader mislabels one of these chemicals as propylene glycol. Drug manufacturers are supposed to test propylene glycol for contamination prior to using it, but that doesn’t always happen.
In response to the contamination episodes that came to light over the past year, Indian drug authorities in June began requiring the testing of cough syrups in a government lab prior to export.
The packaging of the Cold Out obtained in Iraq indicates it was manufactured in January 2022. The WHO has said that it’s exploring whether a spike in prices of propylene glycol contributed to the recent contamination cases. In addition to those linked to Indian medication, an outbreak last year in Indonesia, caused by medication manufactured domestically, killed about 200 children.
Propylene glycol prices tripled in China in 2020 and in India in 2021 and remained elevated for more than a year, according to ChemAnalyst, a market research firm in India. That increased the potential profit from mislabeling a cheaper solvent as propylene glycol.
Valisure is known for finding dangerous chemicals in drugs and personal-care products. Its 2019 research on contamination in the blockbuster heartburn drug Zantac led to recalls and eventual market withdrawal. Valisure works with health-care companies including Kaiser Permanente to test drug products for quality.
--With assistance from Zulfugar Agayev, Onur Ant, Kendall Taggart, Helena Bedwell, Zeke Faux, Debjit Chakraborty, Alexander Gilbert Campbell, Ekow Dontoh, Saritha Rai, Eltaf Najafizada, Eric Ombok, Swati Gupta, Sam Dagher and Nayla Razzouk.
Author: Zachary R. Mider