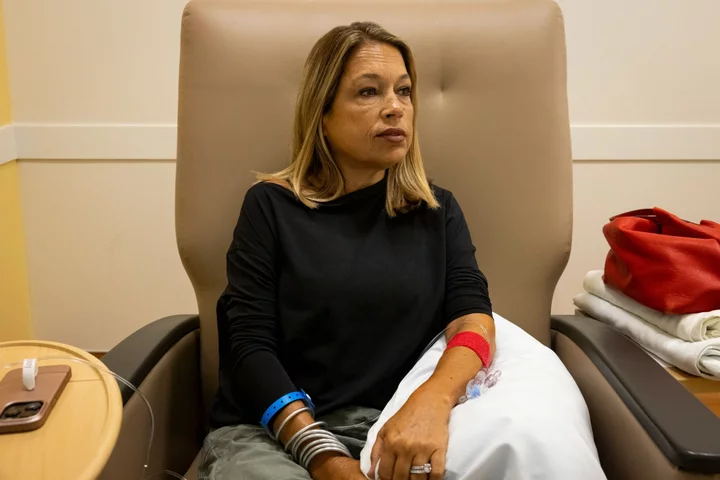
Liany Benedi long dreamed of a cure for the disease that has shadowed her since she was 2 years old.
A year ago, her dream was realized, but getting access to the scientific breakthrough that could change her life forever has become a challenge all its own.
Benedi, now 49, has beta thalassemia, an inherited blood disorder that robs the body of oxygen. In the 1960s, children with the disease would often die before turning 12. Over time, a battery of treatments was developed that could help most people reach middle age — while making them a hostage to frequent blood transfusions and exposing them to other risks.
Enter Bluebird Bio Inc., whose gene therapy Zynteglo was approved in the US last August. Zynteglo is a one-time treatment that aims to correct the flaw that causes beta thalassemia. It works by inserting new instructions into the patient’s blood cells to make more hemoglobin, the protein that carries oxygen around the body. People who received Zynteglo in clinical trials described their lives changing in ways they could never have imagined. They were able to take on more demanding jobs or travel for weeks on end without worrying about receiving more blood.
For Benedi, who sits for hours-long transfusions of healthy blood every three weeks and takes four daily pills to keep her iron levels in check, that sounded like a miracle. In the past 15 years, reactions to donated blood nearly killed her four times. Until the pandemic forced her to stop, she held an annual drive to find new donors whose blood matched hers, to minimize the risk of side effects.
Eager to get Zynteglo, which costs $2.8 million, Benedi connected with Bluebird soon after the approval — but found that her options, for now, are narrow. There are only a limited number of facilities able to administer the treatment to adults, who could face more risks from the complex process required to deliver it.
“I have not stopped crying,” she said.
Read more: Seven-Figure Prices Fair for Rare-Disease Cures, Vertex Executive Says
What Benedi and patients like her are facing reflects a harsh reality of new gene therapies: Not everyone who could benefit will be able to access them, whether because of health complications, high prices for the breakthroughs or living far away from specialized treatment facilities.
The payoff has been slow to arrive for Bluebird as well. When the Somerville, Massachusetts-based biotech reported second-quarter earnings on Tuesday, just 11 people had started the therapy. The company’s stock price has fallen by about 40% since Zynteglo’s approval.
Wider Access
Zynteglo isn’t like most drugs. It involves a procedure where blood stem cells are drawn and sent to a lab for modification. Doctors use a strong chemotherapy called busulfan to clear out the old cells and make room for the modified ones. Once infused back into the patient, the person typically spends weeks in the hospital recovering.
Initially, Zynteglo was available mostly at children's hospitals. M Health Fairview, a hospital affiliated with the University of Minnesota, was the only treatment center listed during the initial phase of the drug's rollout that didn’t specialize in pediatrics, according to a publicly available document. Another site, Boston Medical Center, has since been added. Bluebird says it plans to widen access to more facilities that treat adults.
John Zuke, 51, said he sought the procedure at the Minnesota facility, but was told they wouldn’t treat him because of his age. Zynteglo is intended for people with severe beta thalassemia, according to its label, which doesn’t mention age. But some doctors have said they fear that the rigors of the treatment present risks for older patients who may suffer from other health problems that can be brought on by the disease.
M Health Fairview declined to comment on Zuke’s case, citing privacy laws, but said once its program is fully active, it will provide the treatment according to the best practices that led to its FDA approval, and will consult with every patient who's referred to discuss the risks and benefits.
Zuke said he stopped pursuing the treatment, deciding that possible freedom from transfusions isn’t worth the temporary burden it would place on his family.
Bluebird has long said that Zynteglo’s complexity would result in a gradual rollout. Onboarding a treatment center can take months. Only 15 facilities are currently offering Zynteglo, though another few dozen are expected to open by the end of the year. The company said it intends to build a network that supports patients of all ages, and it anticipates activating multiple adult centers this summer.
Rare Disease
Beta thalassemia is considered rare in the US. Bluebird estimates the most severe form afflicts as many as 1,500 people, most often among those of Mediterranean, South Asian and Middle Eastern descent. People near or in their 50s account for only a sliver of that total.
Benedi’s current treatment regimen can be agonizing. It usually takes five or six needle sticks to find a vein that isn’t hardened from years of transfusions. The week after a transfusion, Benedi says she feels great. But by the second week she is fatigued, and three weeks in, she feels lethargic as her levels of oxygen-rich hemoglobin plummet. The cycle keeps her from working.
Benedi says her blood has developed antibodies that fight off donations that aren’t a perfect match. She prays her body won’t reject new blood, which can cause pain and fever or, in more severe cases, cause her organs to shut down. Her spleen, gallbladder and appendix have been removed. Last year, she was diagnosed with diabetes brought on by her disease.
The US Food and Drug Administration didn’t place any age restrictions on Zynteglo. Although people needed to be under 50 to enroll in the drug’s clinical trials — the oldest patient in the studies was 34 — it was cleared broadly for adult and pediatric patients with beta thalassemia who require regular red blood-cell transfusions.
Bluebird spokesperson Sarah Alspach said that treatment decisions are at the discretion of the treating physician and the company can’t speak to individual patient experiences. The company has heard from some clinicians that they will likely treat patients who are most similar to people in the trials at first, and expand as they gain experience with the therapy, Alspach said.
Children with beta thalassemia have more options for specialized care than adults, even though more people are surviving for longer with the disease. There’s also a shortage of doctors with expertise in anemia, sickle-cell disease and other blood disorders.
“There's this amazing gene therapy, it's a curative option, it has excellent outcomes in all the data to make patients transfusion-independent,” said Farzana Sayani, director of the comprehensive adult thalassemia program at Penn Medicine. “But I do think that the lack of adult thalassemia care in this country has contributed to where we are now in terms of why our 50-year-olds feel they don't have access to this.”
Penn, one of the few adult thalassemia centers in the US, is applying to administer Zynteglo. If approved, Sayani expects Penn to review each case, with age being only one factor.
Being Patient
Not knowing when and where new treatment centers will open has been difficult for Janelle Trieu, a 30-year-old living with beta thalassemia in the Denver area. She contacted Bluebird shortly after Zynteglo was approved and said she was repeatedly told the centers weren’t taking adults.
“It’s frustrating because I want it so badly,” Trieu said in May. “I get that these things take time and I have to be patient, but it feels like I’ve been patient for a long time.”
Later that month, Bluebird put her in touch with UCSF Benioff Children's Hospitals in San Francisco, which was willing to treat her. She said she is still waiting to schedule the next steps.
Alspach, the Bluebird spokesperson, said it’s very encouraging to see the interest and eagerness for gene therapy among adults.
“These are individuals who understand more than anyone the challenges of living with this disease and significant burden that chronic red blood cell transfusions place on a person’s health and well-being over the course of their lifetime,” Alspach said, adding that Bluebird is committed to establishing a qualified treatment center network to meet the needs of all patients.
Benedi and other older patients said they understand why children who could be spared a lifetime of transfusions are first in line.
“I want, obviously, for the young kids to have this, absolutely a hundred percent,” Benedi said. “But I want this for us as well. We don't have that much time left to get this.”